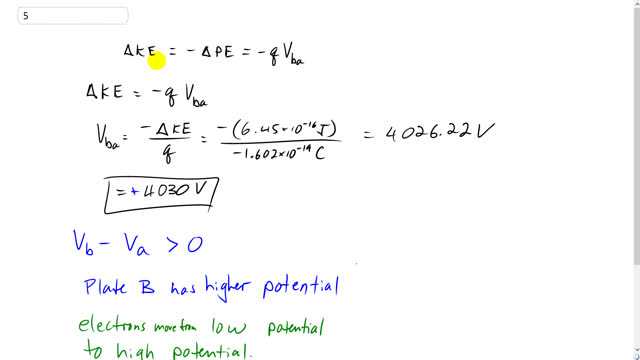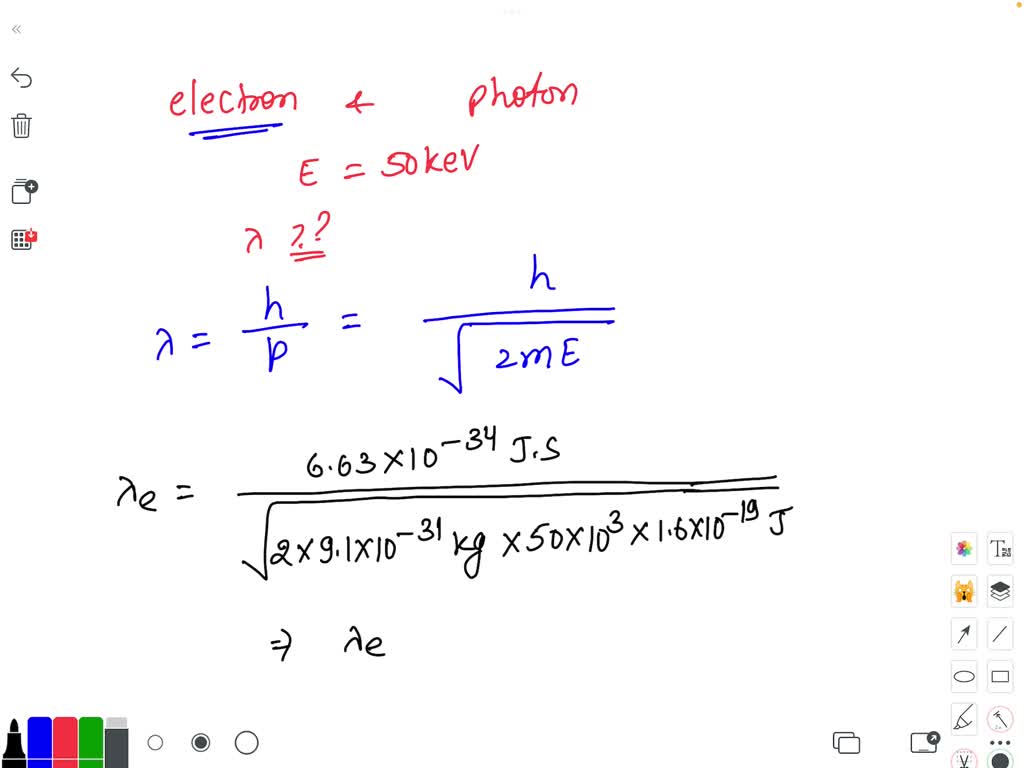
SOLVED: An electron and a photon each have kinetic energy equal to 50 keV. What are their de Broglie wavelengths?
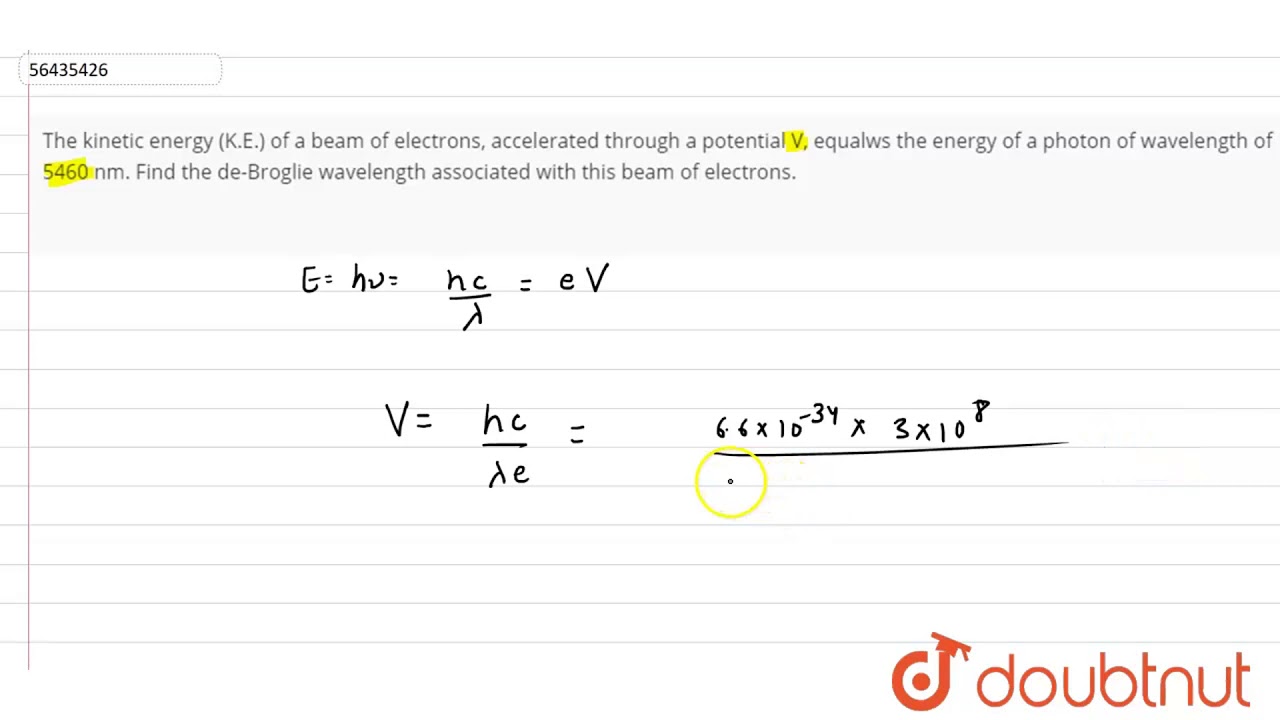
The kinetic energy (K.E.) of a beam of electrons, accelerated through a potential V, equalws - YouTube

Calculate kinetic energy of an electron emitted from a surface of potassium metal (work function = 3.62 × 10^-16J) by light of wavelength 3 × 10^-10 m

What is the kinetic energy of an electron in electronvolts with mass equal to double its real mass? - YouTube
kinetic energy of an electron which is associated with de Broglie's wavelength 20 angstrom is 1)1.0eV, 2)1.51eV, 3)0.59eV, 4)0.38eV.

Particle velocity as a function of kinetic energy for electron (black... | Download Scientific Diagram

Question Video: Calculating the Kinetic Energy of an Electron Moving through a Negative Potential Difference | Nagwa
The (average) kinetic energy of the free electrons (mass = m, |charged| = e), in a metal, at a temperature T in kelvin, equals - Sarthaks eConnect | Largest Online Education Community

If an electron and a proton have the same kinetic energy,the ratio of their de Brogile wavelengths will be - Physics - Dual Nature Of Radiation And Matter - 14107279 | Meritnation.com
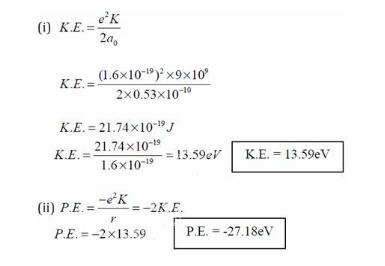
Using Bohr's postulates, obtain the expression for (i) kinetic energy and (ii) potential energy of the electron in stationary state of hydrogen atom. - Sarthaks eConnect | Largest Online Education Community

The Kinetic energy of an electron in the second Bohr orbit of a hydrogen atom is: (a 0 is Bohr radius)
![Solved Problems - Engineering Physics [Book] Solved Problems - Engineering Physics [Book]](https://www.oreilly.com/api/v2/epubs/9788131775073/files/images/co4n066.png)
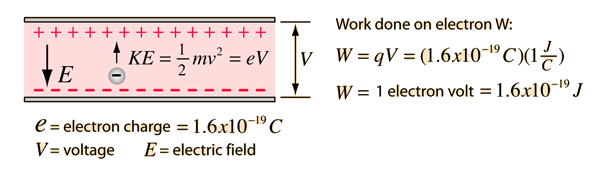






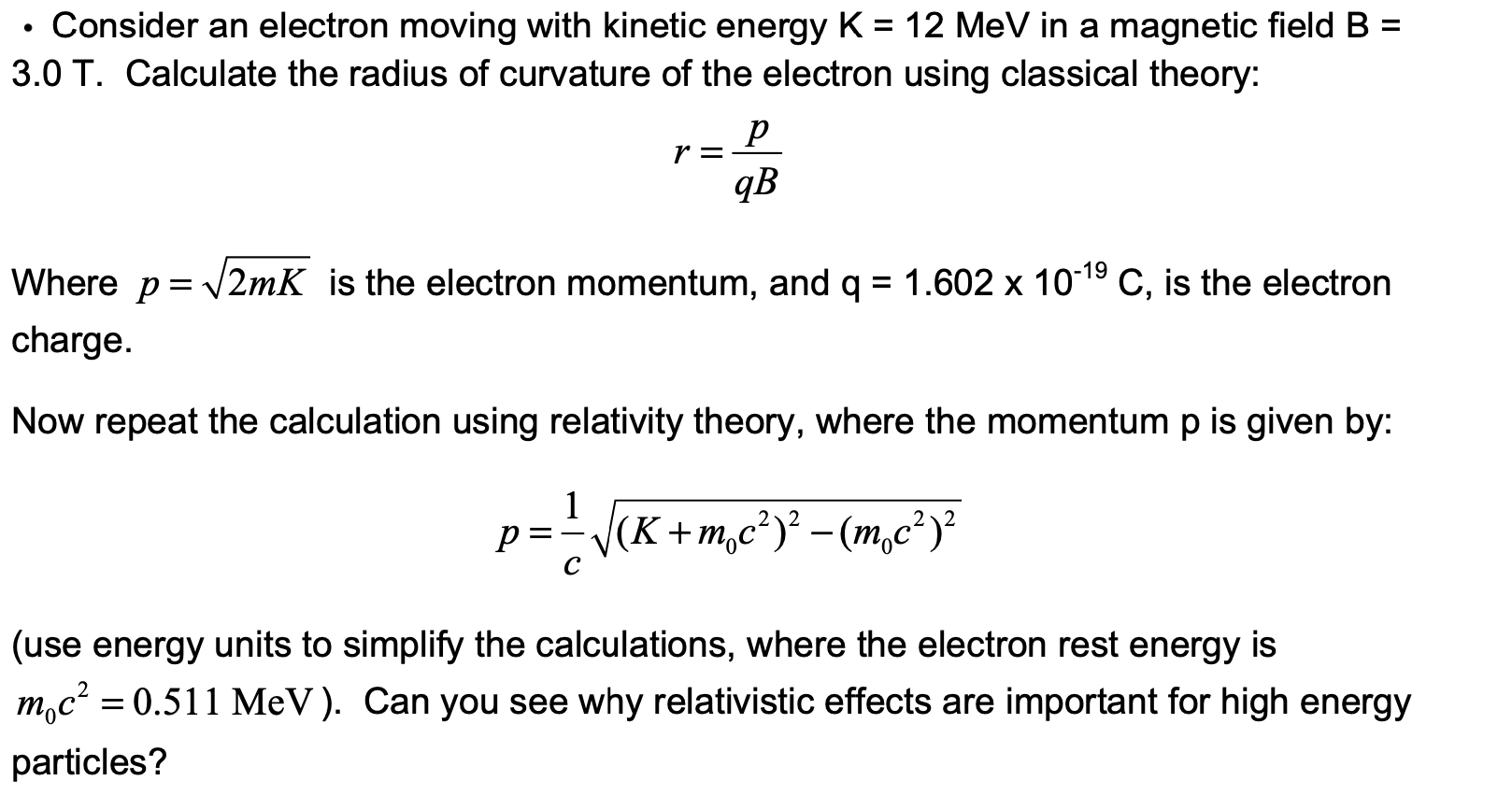
![The kinetic energy of an electron is `4.55 xx 10^(-25)J`. Calculate the wavelength . `]` - YouTube The kinetic energy of an electron is `4.55 xx 10^(-25)J`. Calculate the wavelength . `]` - YouTube](https://i.ytimg.com/vi/He9LjP05EHA/maxresdefault.jpg)