Cipla receives final approval for generic version of Shire's Firazyr | Indiablooms - First Portal on Digital News Management
PREFERRED SPECIALTY MANAGEMENT POLICY Hereditary Angioedema – Icatibant Preferred Specialty Management Policy • Firazyr ® (

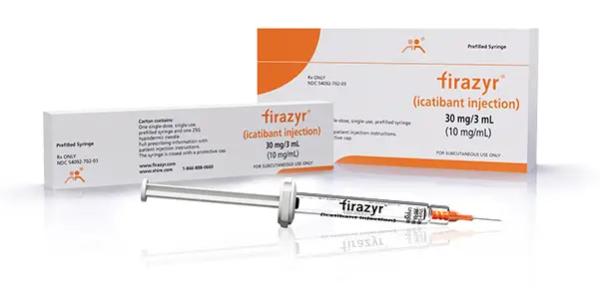
Slayback Pharma announces approval of Icatibant Injection 30 mg/3 ml (10 mg/ ml), generic equivalent of Firazyr®.