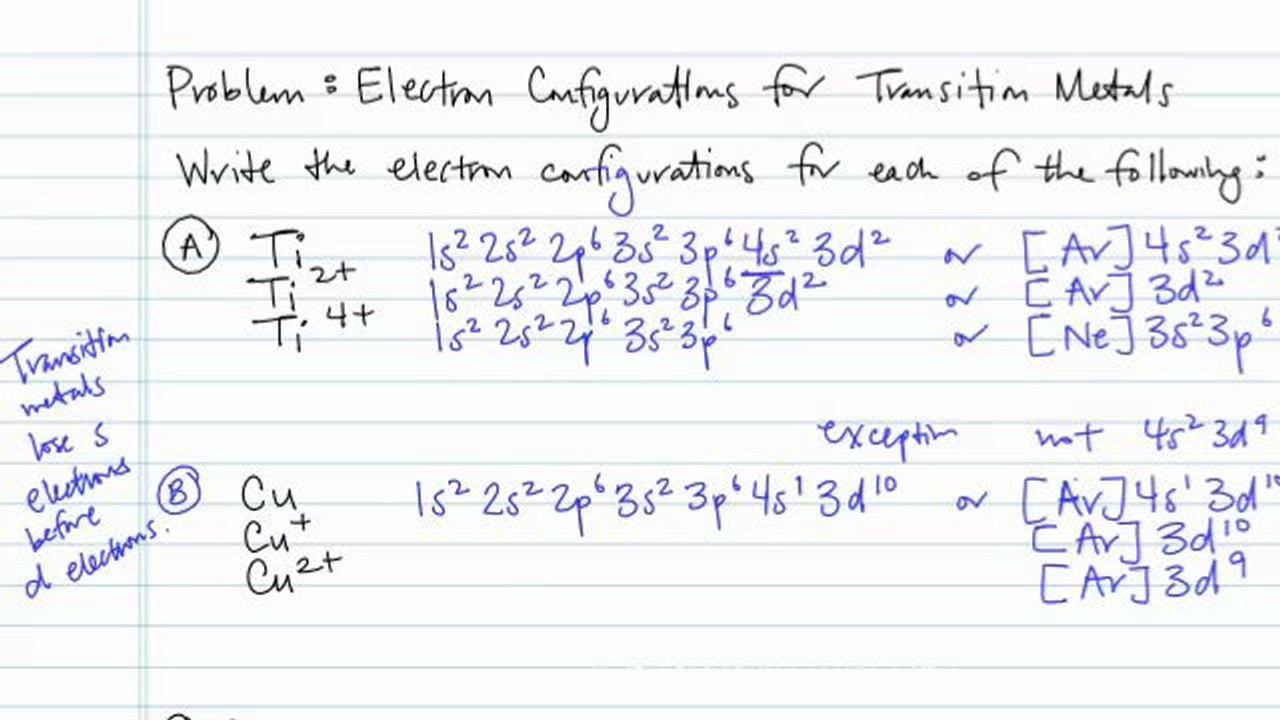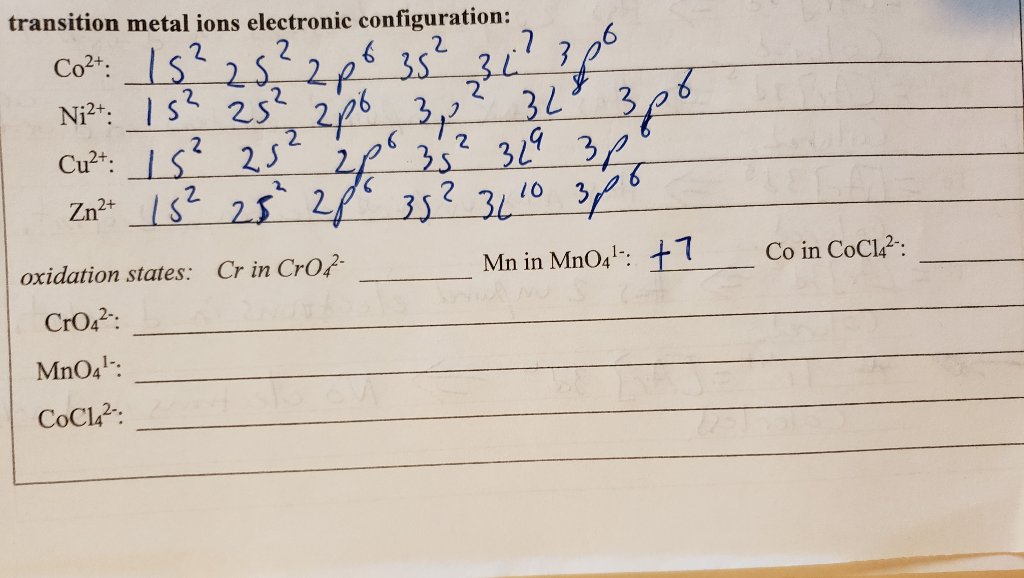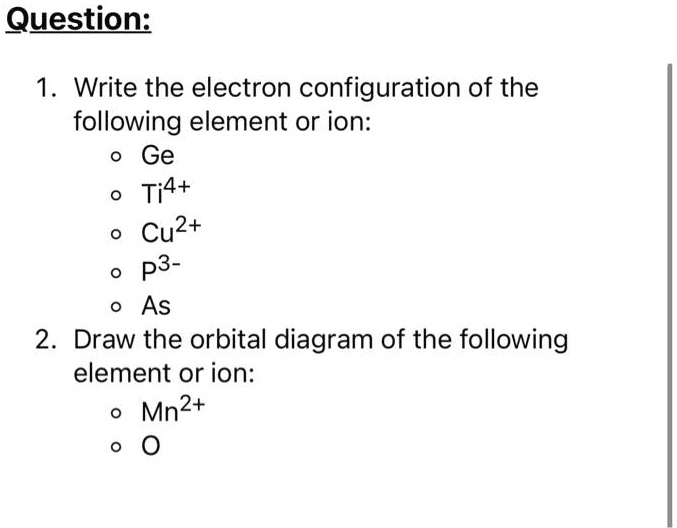
SOLVED: Question: 1 Write the electron configuration of the following element or ion: Ge Ti4+ Cu2+ P3 - As 2 Draw the orbital diagram of the following element or ion: Mn2+

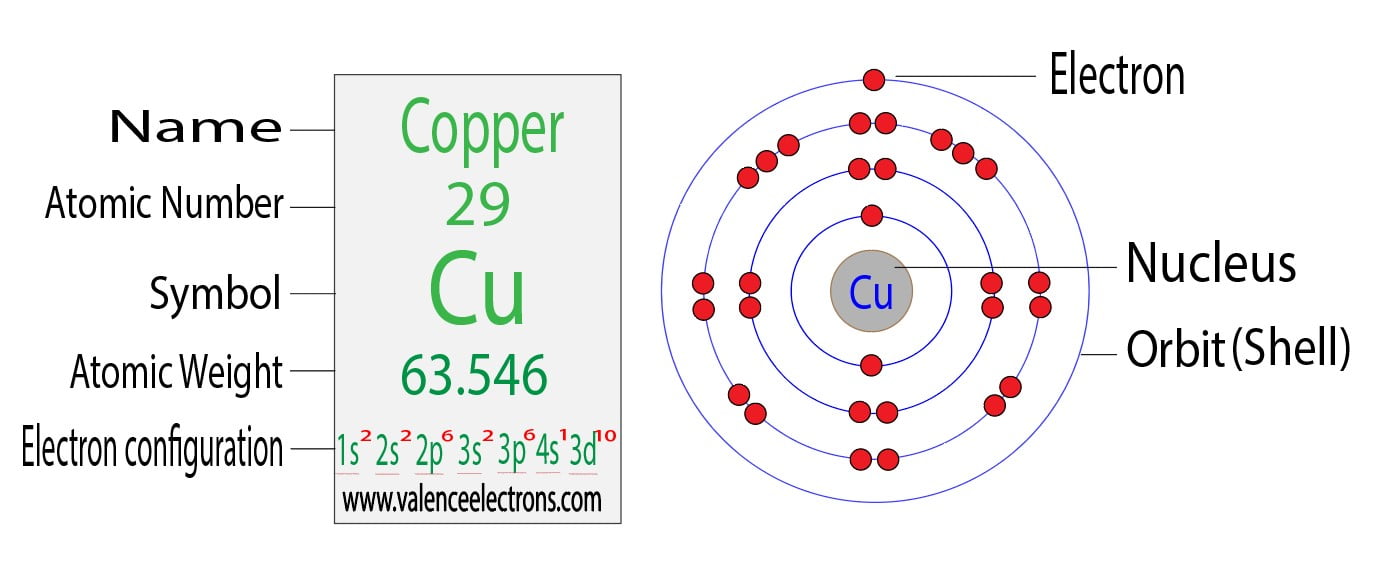
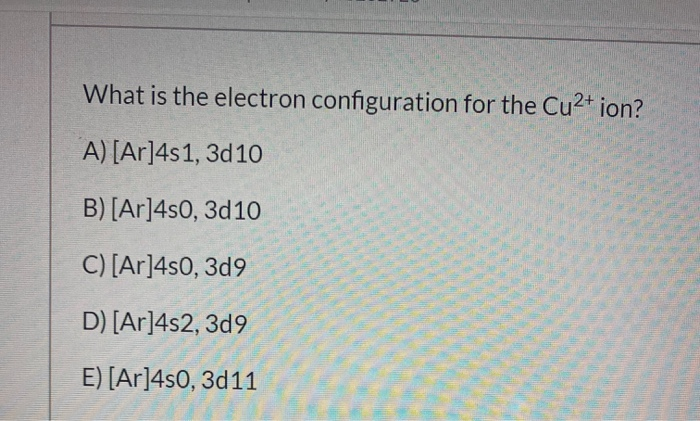
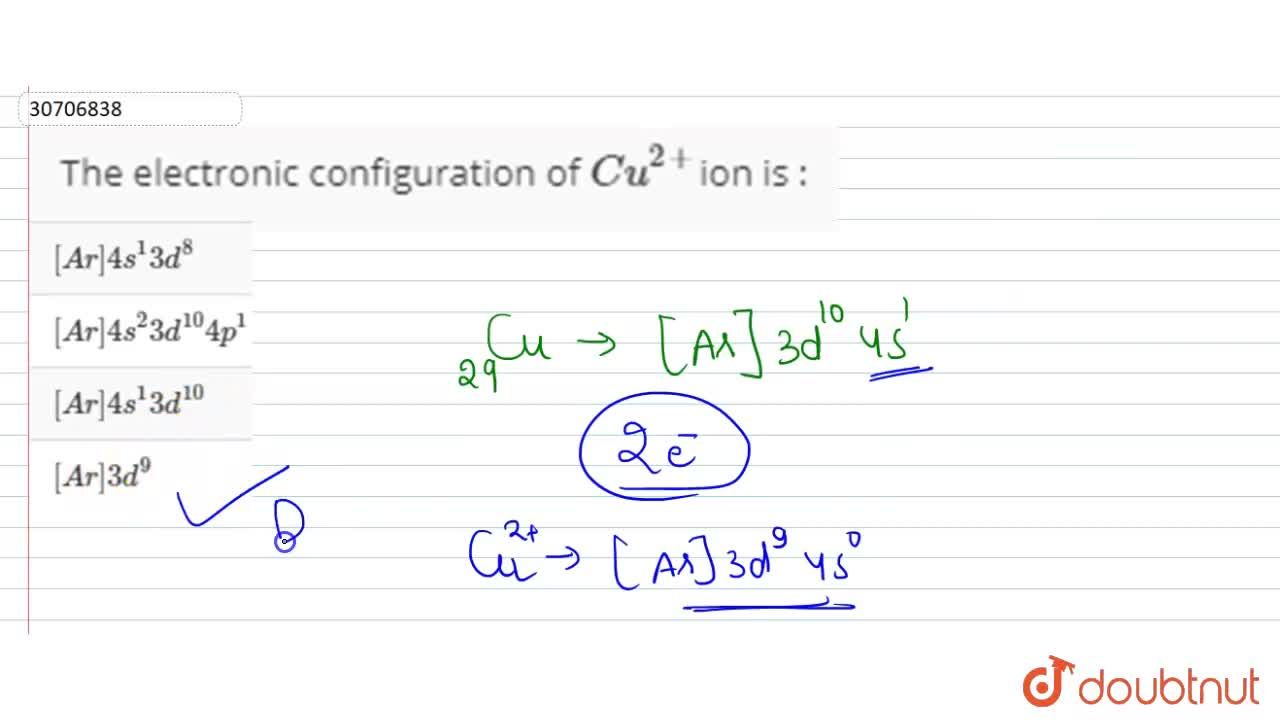
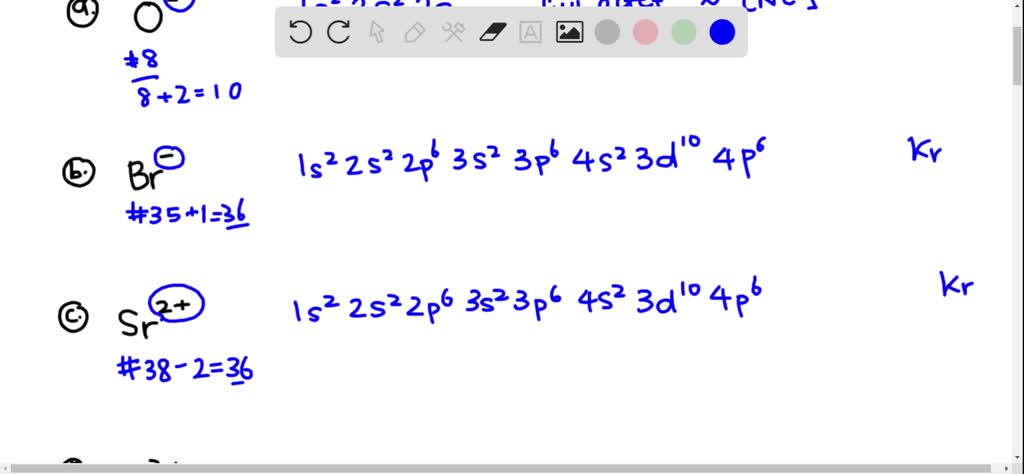
The atomic number of Ni and Cu are 28 and 29 respectively. The electronic configuration 1s^2 2s^2 2p^6 3s^2 3p^6 3d^10 represents:

Copper Cu transition metal Chemistry copper(I) Cu+ copper(II) Cu2+ ion complex ions ligand substitution compounds redox chemical reactions principal oxidation states +1 +2 GCE AS A2 IB A level inorganic chemistry revision

SOLVED: transition metal ions electronic configuration: Co2+: 1s32s2 20 3S 2227 126 Ni2+: Ls2 252,246 342 32 3 Cu2+: LS' 25 35 329 3 2 2 3L 10 Zn2+ dUs- 35 5 Cr in Cr04- Mn in MnO4 47 oxidation states: CrO42 : MnOa CoCl? : Co in CoCl? :